Genotoxicity: Mechanisms, Testing Guidelines and Methods
JUNIPER PUBLISHERS-OPEN ACCESS GLOBAL JOURNAL OF PHARMACY & PHARMACEUTICAL SCIENCES
Authored by Rajendra SV
Abstract
Genotoxicity is one of the major causes for cancer. Genotoxins are agents that can cause the damage of DNA or chromosomal structure thereby causing mutations. It can be chemical or radiation. This damage in the somatic cells will lead to various diseases ranging to cancer whereas the damage to the germ cell will lead to heritable diseases. Better identification and understanding of genotoxins would enable us to prevent the potential damage that can be caused by these genotoxic agents. In this article we discuss about the basic of genotoxicity and the importance of genotoxic studies.
Keywords: Genotoxins, Mutagens, DNA Damage, Chromosomal mutation, Testing guidelines.
Introduction
Genotoxicity is a word used in genetics that describes the possession of substance that has destructive effect on the genetic material of the cell (DNA, RNA) thus affecting the integrity of the cell. Genotoxins are mutagens that can cause genotoxicity leading to the damage of DNA or chromosomal material thus causing mutation. Genotoxins can include chemical substance as well as radiation. Genetic toxicology is the branch of science that deals with the study of agents or substances that can damage the cell’s DNA and chromosomes. It is noted that often genotoxicity is confused with mutagenicity. All mutagens are genotoxic however all genotoxic substances are not mutagenic [1].
Genotoxins can be of the following category depending on its effects [2]:
o Carcinogens or cancer causing agents
o Mutagens or mutation causing agents
o Teratogens or birth defect causing agents
The damage of genetic material of somatic cells may lead to malignancy (cancer) in eukaryotic organisms. Whereas the genetic damage of the germ cells may lead to heritable mutations causing birth defects (Figure 1). Mutations can be of any form; which may include duplication, insertion or deletion of genetic information. These mutations can cause varying range of problems in the host, from a wide variety of diseases to cancer [3,4] One of the best ways to control the damage due to mutagens and carcinogens is to identify the substance or chemical, i.e. antimutagens /anticlastogens (which suppress or inhibit the mutagenesis process by directly acting on the cell mechanism) and demutagens (which destroy or inactivate the mutagens partially or fully thereby affecting less population of cell) from the medicinal plants so that it can be used as antimutagenic and anticarcinogenic food or drug additives [5-7].
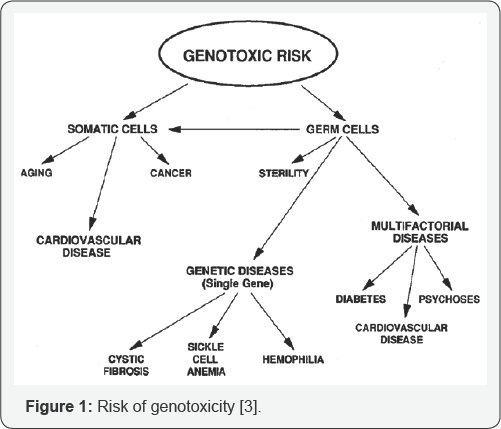
Importance of genotoxicity studies
Genotoxicity studies can be defined as various in-vitro and in-vivo tests designed to identify any substance or compounds which may induce damage to genetic material either directly or indirectly by various mechanisms. These tests should enable the identification of hazard with respect to DNA damage and fixation [8]. Genetic change play only a part in the complex process of heritable effects and malignancy which include the fixation of the damage to the DNA by gene mutation or large scale chromosomal damage or recombination or numerical chromosomal changes. These tests play an important role in predicting if the compound have the potential to cause genotoxicity and carcinogenicity by testing them positive [9]. As a part of safety evaluation process, regulatory authorities all over the globe require information on the genotoxic potential of the new drugs. Genotoxicity is usually evaluated along with other toxicological end points during the safety assessment [10] (Figure 2).
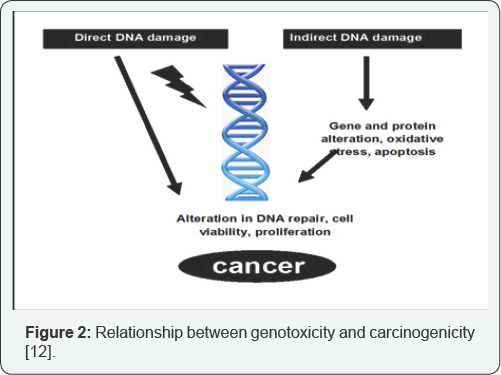
During the early testing stages; the same testing assays are carried out for predicting both the potential heritable germ cell damage as well as the carcinogenicity because these endpoints have common precursors. The relationship between exposure to particular chemical and carcinogenesis has been established whereas such relationship has been difficult to establish for heritable diseases, genotoxic studies have been mainly associated and used for the prediction of carcinogenicity of a compound [11,12]
Classifiaction of carcinogens
EU classification of carcinogens [13]
o Carcinogen category 1-shown to cause cancer in humans
o Carcinogen category 2-causes cancer in animal tests, and most probably also in humans
o Carcinogen category 3-possibly carcinogenic, but evidence supporting carcinogenicity is inadequate for the classification to category 2.
Iarc (International Agency For Research On Cancer) classification of carcinogens [4]
o IARC class 1-The substance is carcinogenic to humans.
o IARC class 2A-The substance is probably carcinogenic to humans.
o IARC class 2B-The substance is possibly carcinogenic to humans.
o IARC class 3-The substance is not classifiable to as to its carcinogenicity to humans.
o IARC class 4-The substance is probably not carcinogenic to humans.
Agents that can cause direct or indirect damage to the DNA
Reactive oxygen species are known to be genotoxic in nature, thus any chemical or substance that may increase the reactive oxygen species (ROS) production might evidently add to the endogenously produced ROS and may lead to non-linear relationships of dose-effect. The following agents are capable of damaging the DNA directly or indirectly; [14]
o Electrophilic species that form covalent adducts to the DNA
o Reactive oxygen species
o Ultra violet and ionizing radiations.
o Nucleoside analogues
o Topoisomerase inhibitors
o Protein synthesis inhibitors
o Some herbal plants like Aconite, Alfa-alfa, Calamus,Aloe vera, Isabghol etc.
Anti-mutagen is any agent that decreases the effect of spontaneous and induced mutations. There are mainly two mechanisms of anti-mutagenesis:
- Desmutagenesis in which the factors on the mutagens are somehow inactivated,
- Bio-antimutagenesis, in which the factors act on the process of mutagenesis or by repairing the damaged DNA that result in the decreased frequency of DNA mutation [15]. Our cells have several DNA repair system by which they try to control the DNA mutations naturally.
The five major pathways through which the cell repair the damaged DNA are: [16-19]
o Direct repair
o Base excision repair( BER)
o Nucleotide excision repair (NER)?
o Mismatch repair
Single/ double strand break repair
Mechanism of genotoxicity
The damage to the genetic material is caused by the interactions of the genotoxic substance with the DNA structure and sequence. These genotoxic substance interact at a specific location or base sequence of the DNA structure causing lesions, breakage, fusion, deletion, mis-segregation or nondisjunction leading to damage and mutation [20]. For example, in its high-valent oxidation state the transition metal chromium interacts with the DNA so that DNA lesions occur leading to carcinogenesis. Researchers have found that the mechanism of damage and base oxidation products for the interaction between DNA and high-valent chromium are relevant to in-vivo formation of DNA damage leading to cancer in chromate- exposed human population, thus making high valent chromium a carcinogen (Figure 3).
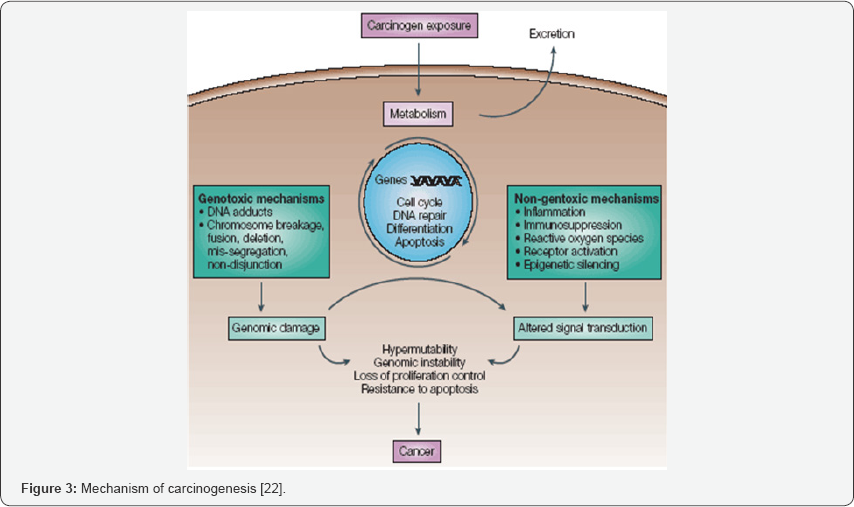
Reactive oxygen species causes one of the most abundant oxidative lesions in DNA and is 8- hydroxydeoxyguanosine (8- OHdG), which is a potent mutagenic lesion. Oxidants as well as free radical when present in the cellular system can adversely affect and alter the structure of lipids, proteins as well as DNA. Reactive aldehydes like 4-hydroxynonenal (4-HNE) are generated by the decomposition of lipid peroxyl radicals or primary free radical intermediate of lipid peroxidation. 4-Hydroxynonenal is involved in many of the oxidative stress related diseases such as atherosclerosis, fibrosis, neurodegenerative diseases it. Many studies have indicated that 4-Hydroxynonenal can stimulate the cell proliferation, differentiation as well as cytoprotective response through its effects on various signalling pathway [21].
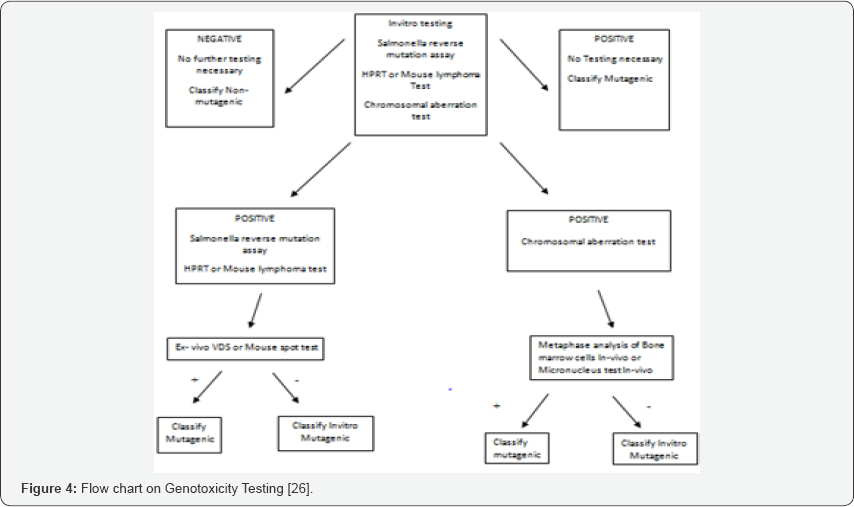
Standard test battery for genotoxicity
The standard test battery for genotoxicity recommends the following for genotoxicity
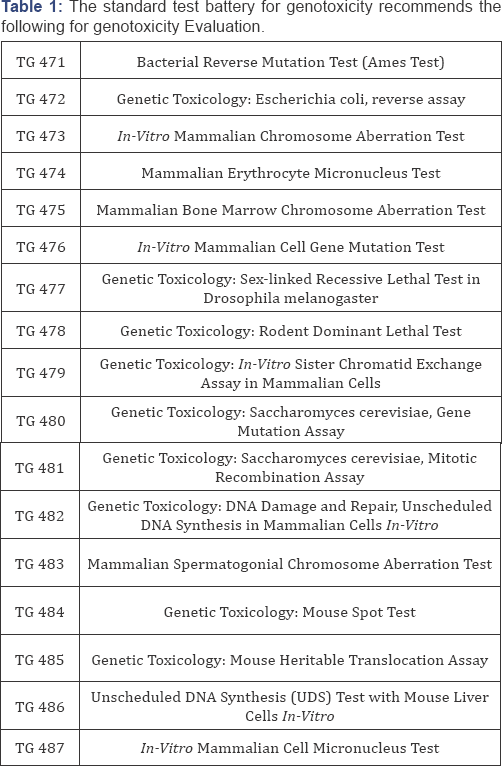
o Testing for gene mutation in bacteria
o In-vitro: cytogenetic evaluation of chromosomal damage with mammalian cells or mouse lymphoma assay.
o In-vivo: test for chromosomal damage using rodent hematopoietic cells (Figure 4).
Purpose of genotoxicity assays
Assays even though inexpensive, have high statistical power and can be reproduced and have the ability to detect a wide variety of genotoxic end-points. It also allows the detection of a drug's potential to cause genotoxicity even in the early stage of drug development. They are designed in such a way that it can be more sensitive to damage so as to enhance the identification of hazard [25,26].
In-Vitro testing methods
There are many in-vitro genotoxicity testing methods available. Some of the commonly used tests which are also a part of the standard battery are [27]:
• Bacterial reverse mutation test which is otherwise called as Ames test whose endpoint is the gene mutations in the bacterial cell [28-29].
• Mammalian chromosome aberration test with the end point of chromosome aberration [30-31].
• Mammalian cell gene mutation test or the mouse lymphoma test whose end point is the gene mutations [32,33].
Bacterial reverse mutation test: The Bacterial reverse mutation test was developed by Ames. B thus the name Ames test. The amino acid requiring strains of Salmonella typhimurium and Escherichia coli are used in the bacterial reverse mutation test to detect the mutation points which may involve substitution, deletion or addition of one or few of base pairs of DNA [34]. The main principle of the test is that after identifying the mutation it reverts it back and restore the functional capability of the mutant cell to synthesize Histidine. In this test the reverent bacteria cells are identified by the ability of the parent test strain to grow in the absence of amino acids. The bacterial reverse mutation test being rapid, inexpensive and easy to perform is commonly used as an initial screening test for genotoxicity or mutagenicity [35,36].
Mammalian chromosome aberration test: The main purpose of the mammalian chromosome aberration test is to identify the agents which can cause structural mutations in chromosomes or chromatids, chromatid mutation being the common [37,38]. Other type of chromosomal changes like polyploidy and duplication can also be found using this test. A positive test result shows a potential mutagenic or carcinogenic of the agent but there is not a perfect correlation [39].
Mammalian cell gene mutation test: This test is used to find the gene mutations caused by the chemical substances. The commonly used cell lines include L5178Y mouse lymphoma cells, the CHO, CHO-AS52 and V79 lines of Chinese hamster cells, and TK6 human lymphoblastic cells [40-41]. It detects the end points like thymidine kinase (TK) and hypoxanthine-guanine phosphoribosyltransferase (HPRT), and a transgene of xanthine- guanine phosphoribosyltransferase (XPRT) mutation [42,43].
In-vivo genotoxicity testing methods
The in-vivo genotoxicity test or assays are done supplemental to in-vitro assay if an in-vitro positive result is obtained. Some of the in-vivo tests done are as follows [44].
In-vivo comet assay: It is one of the commonly used in-vivo test used for hazard assessment of agents which have potential for genotoxicity or mutagenicity [45]. It helps in detecting the DNA damage and detects a broad variety of primary DNA lesions which cannot be identified by any other tests. This test can be applied to a wide variety of tissues or any special cell types. Being sensitivity to even low level of DNA damage it requires only small amount of cells per sample and it can be completed in a short period of time [46].
In-vivo micronuclei test/In-vivo chromosome aberration test: It is a test done to identify the damage done chromosome or spindles. On exposure to the mutagen the cell may undergo damage and on division it will form smaller micronucleus additional to the main nucleus [47].
Conclusion
Genotoxins are agents that can interact with the DNA thus causing mutations and damaging its structure and may lead to cancer. They act by changing the chromosomal structure by addition, deletion, duplication, forming rings etc. The mutations may lead to a wide variety of diseases to cancer. It is very important to do genotoxicity studies so as to avoid the potential damage that can be caused by it. These genotoxicity tests are done to identify if a drug or other substance have the potential to cause mutation and genotoxicity. By doing so they help us identifying the hazards in the early stage of drug development itself. Identification of the genotoxic agents helps us understand the mechanism of the mutation and genotoxicity thereby paving us way to better prevent the frequency of such mutation and genotoxicity.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com
For more articles in Global Journal of Pharmacy & Pharmaceutical Sciences please click on: https://juniperpublishers.com/gjpps/index.php


Comments
Post a Comment