Amylase & Lipase Inhibitory Effects and Antioxidant Effects of Novel Oxazolines (Lam.) Verdc (Kulattha): A Conceptual Study-Juniper publishers
JUNIPER PUBLISHERS-OPEN ACCESS GLOBAL JOURNAL OF PHARMACY & PHARMACEUTICAL SCIENCES
Most of the studies reveal the inference of oxidative
stress in diabetes pathogenesis by the alteration in enzymatic systems,
lipid peroxidation, impaired Glutathione metabolism and decreased
Vitamin C levels. By seeing the significance of Amylase & Lipase
inhibitory effects and antioxidant effects in reducing the burden of
Diabetes, herewith aimed to synthesize oxazolines and to screen for
Amylase & Lipase inhibitory effects and antioxidant effects. Twenty
five Oxazoline derivatives are prepared, characterized and screened for
the anti oxidant, lipase and amylase inhibitory activity. Out of 25
synthesized compounds seven compounds shows significant amylase
inhibitory activity, twelve compounds shows significant antioxidant and
lipase inhibitory activity. Dimethoxyl group, halogen or nitro group at
para position and dimethyl amino substitution enhance amylase inhibitory
effect. Most of the compounds exhibited lipase inhibitory effects have
significant anti-oxidant action.
Keywords:Oxazolines; Pancreatic lipase; AmylaseIntroduction
Oxazoline play a major role in medicinal chemistry.
These are five membered heterocycle with one double bond and oxygen and
nitrogen as hetero atoms. There are many natural and synthetic molecules
which contain oxazoline nucleus having biological significance. The
nitrogen present in Oxazoline is basic in nature. The synthesis,
structures and biological activities of oxazoline derivatives have long
been focused of research interest of organic chemists in the field of
medicine due to the potential biological activities exhibited by them
[1]. Alpha-amylases hydrolyze complex polysaccharides to produce
oligosaccharides and disaccharides which are then hydrolyzed by
α-glucosidase to monosaccharides which are then absorbed through the
small intestines into the hepatic portal vein. Inhibitors of α-amylase
and/or α-glucosidase such as acarbose, miglitol and voglibose [2] are
known to lower postprandial hyperglycemia (PPHG) in type 2 diabetic
patients by delaying digestion and subsequent absorption of
carbohydrates from the gut. Newer approaches for the treatment of
obesity have involved inhibition of dietary triglyceride absorption via
inhibition of pancreatic lipase.
Prevention of digestion of digestive lipids could be
an effective strategy for preventing systemic absorption of lipids. Any
imbalance between the free radicals and antioxidants leads to produce a
condition known as “oxidative stress” that results in the development of
pathological condition among which one is diabetes. Most of the studies
reveal the inference of oxidative stress in diabetes pathogenesis by
the alteration in enzymatic systems, lipid peroxidation, impaired
Glutathione metabolism and decreased Vitamin C levels. Lipids, proteins,
DNA damage, Glutathione, catalane and superoxide dismutase are various
biomarkers of oxidative stress in diabetes mellitus. Reactive oxygen
(ROS) and nitrogen (RNS) species include superoxide (O2•−), hydrogen
peroxide (H2O2), hypochlorite (ClO−), hydroxyl radical (OH•), nitric
oxide (NO), and peroxynitrite (ONOO−) [3]. In physiological conditions,
mitochondria are the major site of intracellular ROS production, due to
electron leakage along the respiratory chain; nevertheless, they can
also arise from plasma membrane systems, endoplasmic reticulum,
lysosomes, peroxisomes and cytosolic enzymes.
At low concentrations, ROS/RNS exert a multitude of
biological effects, including immune-mediated defense against pathogenic
microorganisms and intracellular signaling; conversely, high levels of
these extremely reactive species can damage DNA, lipids, and proteins,
thus leading to tissue injury and cell death Endogenous antioxidant
compounds are urate, glutathione, ubiquinone, and thioredoxin;
furthermore, some proteins (ferritin, transferrin, lactoferrin,
caeruloplasmin) act as antioxidants, as they bind and sequester
transition metals that may start oxidative reactions. Antioxidant
enzymes are superoxide dismutase (SOD), glutathione peroxidase (GPx),
glutathione reductase, glutathione S-transferase, catalase,
thioredoxin reductase, peroxiredoxins (Prx), and NAD(P)
H:ubiquinone oxidoreductase (NQO1). Obesity may develop
oxidative stress and it is the major cause of metabolic syndrome.
Here we are making an attempt to find a lead molecule with
desired effects like anti-amylase, anti-lypase and anti-oxidant
effects.
Experimental
Preparation of sample
Proposed compounds prepared by making reaction between
substituted aromatic aldehydes and dimethylamine in presence
of Pyridinium Hydrobromide PerBromide. The resultant
mixture is washed with sodiumthiosulphate and successively
with sodium chloride. The resultant product recrystallised from
ethanol. The structure of obtained compounds characterized and
screened for anti-amylase, anti-lipase and anti-oxidant effects.
Evaluation of Lipase Inhibitory Activity
Pancreatic lipase inhibitory properties have been extensively
examined for the determination of the potential effect of these
oxazoline derivatives as antiobesity agents. Acinar cells of
pancrease secrete an enzyme pancreatic lipase and it releases
fatty acids from the triglyceride skeleton at the C-1 and C-3
position. These fatty acids are incorporated into bile acidphospholipid
micelles and further absorbed at the small intestine,
and finally enter to the peripheral circulation as chylomicrons.
Interference with fat hydrolysis leads to decreased utilization of
ingested lipids; hence, lipase inhibition reduces fat absorption.
Lipase activity was assayed by turbidimetric method using olive
oil-ethanol suspension. This suspension was prepared by mixing
1 ml of olive oil to 100 ml of ethanol and shaken vigorously. 1mL
of this olive oil-ethanol suspension was added to 9 mL of 0.05 M
Tris-HCl buffer, pH 8.0 containing 0.025M sodium deoxycholate.
This emulsion was used as substrate. Reaction mixture
containing enzyme and inhibitor (in requisite amount) was
incubated at room temperature for 10 min. Reaction was started
by addition of 1 ml of substrate. Incubated for 10 min at 37°C.
The decrease in turbidity was measured at 660 nm. Inhibitors
present in the reaction prevented the decrease of turbidity of the
mixture. Suitable ‘control’ tubes were run parallel [4].
Evaluation of anti oxidant activity
Human body is rich in free radical. The ability of oxazoline
derivatives to scavenge hydrogen peroxide was determined
according to the method of Ruch. A solution of 40 mM hydrogen
peroxide was prepared in phosphate buffer (pH 7.4).100 μg / ml
prepared compound in distilled water were added to a hydrogen
peroxide solution (0.6 mL, 40mM). Absorbance of hydrogen
peroxide at 230 nm was determined after 10 minutes against
a blank solution containing the phosphate buffer without
hydrogen peroxide [5]. % Scavenged [H2O2] = [(AC– AS)/AC] x 100
Here AC and AS are the absorbance of control and absorbance of
test/standard respectively.
In –Vitro Alpha-amylase inhibitory assay
Alpha amylase and alpha glucosidases are responsible
for the breakdown of oligosaccharides/disaccharides to
monosaccharide. So inhibitor of this enzyme delay carbohydrate
metabolism which results in marked decrease in glucose
absorption. To 500 μl of (100μg/ml) test compounds, 500 μl of
starch in phosphate buffer (pH 6.9) containing 6.7mM of sodium
chloride was added. The reaction was initiated by adding 500
μl porcine pancreatic amylase and incubated at 37ºc. To the
above mixture add 1ml of DNSA (1g of DNSA, 30g of sodium
potassium tartarate and 20 mL of 2N sodium hydroxide) was
added and made up to a final volume of 100 mL with distilled
water and kept it in boiling water bath for 5 minutes and cooled
to room temperature. The reaction mixture diluted with 10 ml of
distilled water and absorbance was read at 540 nm. Blank tubes
were prepared by replacing the enzyme solution with 500 μL in
distilled water. Control, representing 100% enzyme activity was
prepared in a similar manner, without sample. Maltose is used
as standard [6].
Inhibition (%) = (Abs of Control – Abs of test) X100
Abs of Control
Statistical Evaluation
All results were expressed as mean ± standard deviation
(n=3). Significance of difference from the control was
determined by dunnet test (one way ANOVA), Twenty five
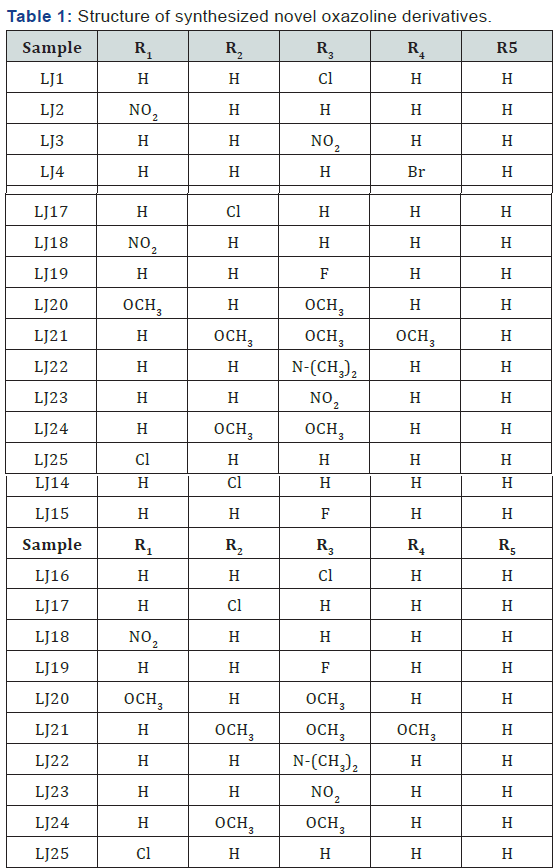
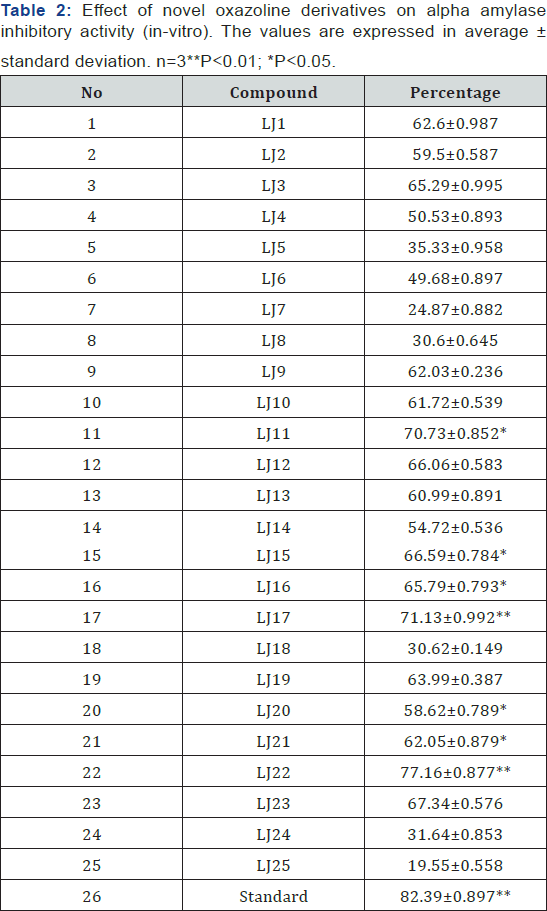
Oxazoline derivatives are prepared, characterized and among
the tested compounds LJ11, LJ12, LJ15, LJ16 LJ17, LJ22, LJ23 found to
have maximum Amylase inhibitory effects (Figure 1 & 2). LJ2,
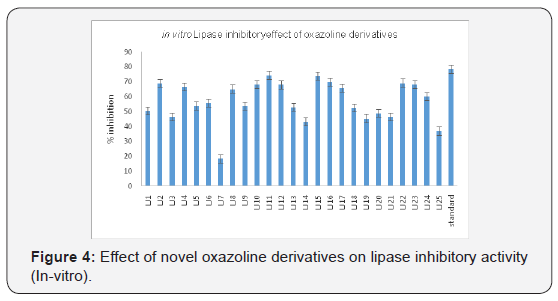
LJ4, LJ8, LJ10, LJ11, LJ12, LJ15, LJ16, LJ17, LJ22, LJ23, LJ24 found
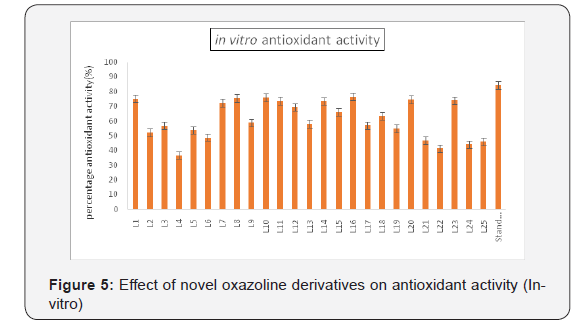
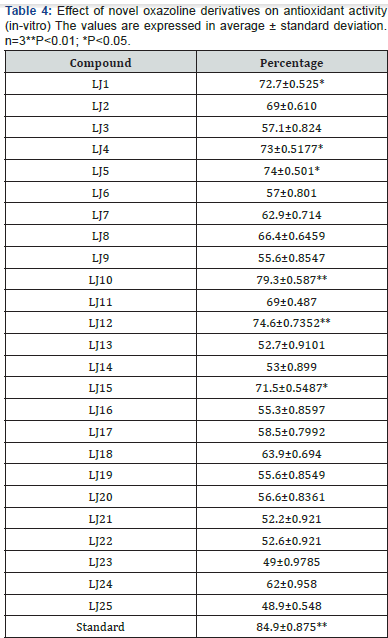
to have maximum lipase inhibitory activity (Figure 3). LJ1, LJ7,
LJ8, LJ10, LJ11, LJ12, LJ14, LJ15, LJ16, LJ18, LJ20, LJ23 (Figure 4)
found to have maximum anti oxidant activity. Amylase inhibitory
effects, that may be attributed to the presence of hydroxyl/
methoxyl/halogen/ nitro/dimethylamino functional groups
group at 3rd/4th position in phenyl ring. The compounds with
more lipase inhibitory effects didn’t show any specific pattern of
presence of certain functional groups. In the case of antioxidant
activity screening phenyl ring attached with halogenated/
methylated/ hydroxylated showed promising activities.

Results and Discussion
Twenty five Oxazoline derivatives are prepared, characterized
and among the tested compounds LJ11, LJ12, LJ15, LJ16 LJ17, LJ22, LJ(Table
1 & 2) found to have maximum Amylase inhibitory effects (Figure
1 & 2). LJ2,LJ4,LJ8,LJ10,LJ11,LJ12,LJ15,LJ16,LJ17,LJ22,LJ23,LJ24
found to have maximum lipase inhibitory activity (Figure 5).
LJ1, LJ7, LJ8, LJ10, LJ11, LJ12, LJ14, LJ15, LJ16, LJ18, LJ20,
LJ23 (Table 3) (Figure 4) found to have maximum anti oxidant
activity. Amylase inhibitory effects, that may be attributed to the
presence of hydroxyl/methoxyl/halogen/ nitro/dimethylamino
functional groups group at 3rd/4th position in phenyl ring. The
compounds with more lipase inhibitory effects didn’t show any
specific pattern of presence of certain functional groups. In the
case of antioxidant activity screening phenyl ring attached with
halogenated/ methylated/ hydroxylated showed promising
activities (Table 4).

Conclusion
There are many natural and synthetic molecules which
contain oxazoline nucleus having biological significance. The
synthesis, structures and biological activities of oxazoline
derivatives have long been focused of research interest of organic
chemists in the field of medicine. Due to the potential biological
activities exhibited by them. There is some sort of association
observed between Anti-oxidant and Lipase inhibitory effect.
Among the substituent’s Dimethoxyl, Dimethylamino and
halogens enhanced Amylase inhibitory effect.
Acknowledgement
It is an outcome of funded project from DST-SERB, India.
Spectral characterization done at SAIF-Cochin.
Author Biography
Dr.Lincy Joseph has completed her PhD in Pharmaceutical
Sciences from Vinayaka Mission University .She is currently
working in Pushpagiri college of Pharmacy, Kerala-India as
Professor in Medicinal Chemistry. She has published more
than 150 papers in reputed journals .She is a member of
many professional bodies of Pharmaceutical Sciences. She has
presented research papers abroad. She has authored textbooks
too. She got awarded as eminent teacher and Best teacher
Pharmacist recently.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com
For more articles in Global Journal of Pharmacy & Pharmaceutical Sciences please click on: https://juniperpublishers.com/gjpps/index.php
To know more about Juniper Publishers please click on: https://juniperpublishers.business.site/


Comments
Post a Comment