Where We Stand In the Global Eradication of Polio- Juniper Publishers
JUNIPER PUBLISHERS-GLOBAL JOURNAL OF PHARMACY & PHARMACEUTICAL SCIENCES
Authored by Ternullo S
Abstract
Despite almost Three decades of progress and the availability of effective vaccines, global WPV eradication has remained elusive. The end game eradication strategy was developed in 2013 and implementation has begun. One dose of IPV was incorporated into routine immunization schedules in fall of 2015 followed by withdrawal of tOPV and its replacement by bOPV for routine use globally in April 2016. Continued challenges exist as the 2018 target date for eradication approaches. Challenges include technical challenges for maintenance of cold chains for surveillance and assurance of OPV potency, warfare and security challenges, hostility toward vaccinators, low literacy rates, politically motivated misinformation, and religious misinformation resulting in vaccine refusal. The lack of accurate health information via government and media channels at a grass roots level and a lack of reliable governmental commitment to immunization and building network infrastructure have also presented challenges. Successful eradication will require addressing these challenges.
The post-eradication plan includes the ultimate withdrawal of all live-attenuated polio vaccines (OPV) for routine use at the specified trigger points, routine reliance on IPV, maintenance of adequate global stockpiles of appropriate vaccines in case of outbreaks, development of pharmaceutical antiviral for polio, and planning for an appropriate response in the event of a WPV or VDPV escape from a storage or production facility. We are on the cusp of a polio-free world but continued progress requires cooperation of parents, community and religious leaders, governments and political leaders at all levels, international technical and healthcare agencies, and major aid donors.
Keywords: Polio; Immunization; Eradication; Immunization; Epidemiology; WHO; Vaccination
Introduction
Eradicable infectious agents are those where the chain of transmission can be broken by an intervention and where there is no reservoir such as a chronic or persistent carrier state, chronic infection, or a non-human host. The only disease eradicated globally is small pox, with the last case recorded in Somalia in 1977. Despite two highly effective vaccines and a disease restricted to humans with very rare cases of chronic persistent infections, the eradication of polio has been elusive. The less favorable geopolitical climate during the polio eradication effort, characterized by multiple global conflicts, as compared to the Cold War smallpox eradication era has contributed to the difficulties encountered [1]. The Global Polio Eradication Initiative (GPEI) was launched at the World Health Assembly (WHA) in 1988 and signaled an international push for a polio-free world by the year 2000.
Epidemiology of polio
Polio has affected populations for millennia and polio- induced paralysis has been found depicted in Egyptian hieroglyphs [2]. Outbreaks were sporadic during the 1880s in the U.S. and Scandinavia until the turn of the century when epidemics began in Scandinavia followed by other European countries and the United States. Unlike other communicable diseases, polio flourished in areas with advanced sanitation and has been referred to as "the middle-class plague". Ever worsening epidemics were characterized by their unpredictability and in temperate climates were strongly seasonal and typically occurred between August and October. There was a striking latitudinal gradient in the timing of epidemics across the temperate climates, but in tropical areas, no such gradient or seasonality existed. The magnitude and regularity of the outbreaks increased in many countries from the pre-WWII era to the post-WWII baby boom area, hitting a peak in the developed world during the 40s and 50s. Polio was largely eradicated in these developed countries through mass immunizations by the 1980s [3,4].
Since before 1955 there has been evidence that some pediatric injections and procedures could induce polio infection and paralysis. Tonsillectomies were documented to increase the risk of bulbar polio. Injection-induced polio was well documented in Italy, Germany, and France in children being vaccinated or injected with antibiotics. In the United States the increase in the incidence of polio roughly correlated with an increase in the number and frequency of immunization programs and paralysis developed in the limb injected with DPT vaccine [5]. Booster shots were sometimes suspended in the U.S. during epidemics. The concern resurfaced with the expanded vaccinations against polio during the 1980s and health care workers began reporting this phenomenon again after vaccination against common childhood diseases. A case- controlled study in Romania demonstrated that the elevated risk of vaccine-associated paralytic polio (VAPP) between 1970 and 1994 was not a result of vaccine components but resulted from the administration of multiple intramuscular injections of antibiotics within 30 days of receipt of live-attenuated oral polio vaccine (OPV).
The chronologic proximity of injections increased the risk of paralysis by a factor of 2-10 times [6]. Several epidemics erupted in India during the 1990s providing new clinical evidence. Tissue injury by an injection assisted the poliovirus' (PV) systemic spread and entry into the spinal cord. Therefore, until wild poliovirus (WPV) was eradicated in endemic regions, polio vaccination needed to be undertaken before other pediatric immunizations to reduce the risk of provoking polio.
Clinical aspects of polio
Polio presents difficulties in recognizing infections since between 95% and 99% of patients are asymptomatic; surveillance and monitoring. About 24% of patients have minor non-specific symptoms such as fever, sore throat, upset stomach, or flu-like illness. Between 1% and 5%, of patients, develop an aseptic meningitis with stiffness of the legs or paresthesias once the minor symptoms resolve. Only about 2% of people experience viral replication in the CNS. Typically, there is complete recovery within 2-10 days. Fever than 1% of polio cases result in limb paralysis. Respiratory paralysis resulting in death occurs in 5%-10% of those patients. The rates of paralysis increase substantially with age. Paralysis occurs in 1/1000 cases for infants but 1/75 cases for adults.
Historically, in areas with poor sanitation, enteroviruses, including polio, were prevalent and infected infants between 6 and 12months of age, These infants were still partially protected through passively acquired immunity resulting in a lower incidence of paralysis. Speculation on the epidemics that occurred in well-developed countries during the 1950s has surrounded the observation that with improved hygiene, the age of first WPV infection was postponed in children past 12 months. At that age, the child had neither passive immunity from mother nor active immunity against the enterovirus, resulting in a higher incidence of paralysis than in younger children [3].
The World Health Organization (WHO) estimates that there are 10-20 million survivors' worldwide living with various disabilities after acute poliomyelitis. In the developed world, most of these survivors are aged 50 or older [2]. The socioeconomic consequences of polio can be profound. When compared with controls that had never had polio, a higher percentage of paralytic polio survivors remained childless, were unemployed despite a higher educational level, were more likely to receive a disability pension, and had higher health costs whether or not continued paralysis was present [7]. Post-polio syndrome (PPS) affects between 25% and 40% of all polio survivors, both those immediately disabled during the acute stage of the disease and those who recover with few or no residual symptoms but who begin to experience new or worsening disabling symptoms after years of stability. The risk of PPS is greatest for females, those with respiratory disturbances during the acute phase of the disease, and those who required the use of orthoses and aids during the recovery period [8].
Progress toward global eradication of polio
More than 60 years after the development of the first vaccine, the disease has still not been eradicated globally, though progress has been made and polio is on the verge of eradication (see Appendix 1 for timeline). Polio was generally eliminated within developed countries within 25-30 years of the first polio vaccine [9]. Target dates for global eradication were missed in 2000, 2012 and 2015 and the current target for eradication is 2018. Despite our failure to eradicate polio completely, GPEI achievements have been impressive. Polio rates have decreased from an estimated 350,000 cases of WPV yearly at the launch of the campaign in 1988 to only 21 cases through August of 2016. The global polio burden is less than 0.02% of the burden reported in 1988 when the GPEI was launched [10]. Of the WPV cases reported in 2016, 19 were reported in the only remaining endemic countries: Afghanistan and Pakistan. The remaining 2 cases were reported in Nigeria and based on mutation viral typing, had been circulating undetected for several years, indicating gaps in the country's surveillance system.
Available vaccines
Salk vaccine (IPV)
The Salk vaccine is considered the safer of the two available vaccines since its trivalent strains are killed and it is effective in inducing humoral immunity without risk of live viral shedding or mutations that could produce VAPP. It is considered the vaccine of choice in areas where polio has been eradicated for long periods of time. Most developed countries utilize only IPV for routine immunization and the United States converted to routine use of IPV in 1997, approximately 18 years after the last reported case of polio in the United States. IPV is not effective in inducing mucosal immunity and therefore has a limited role during polio outbreaks. It is also limited in its ability to produce secondary immunity in contacts of the vaccinated individual since no live virus shedding occurs in stools. Its role in the rapid control of outbreaks and eradicating WPV in endemic countries is minimal when compared to OPV (Table 1).
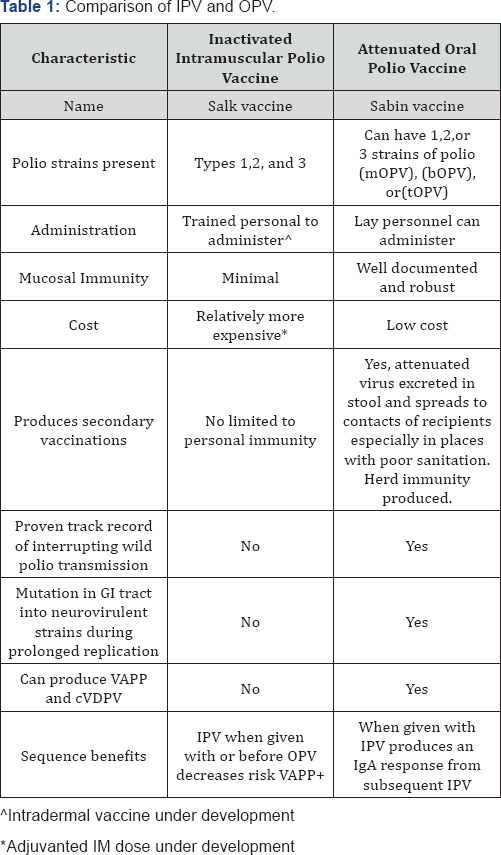
Switching to IPV alone in a region where there is continued circulation of WPV is a risky strategy and its exclusive use is limited to countries where polio has been eradicated and reliable environmental surveillance programs are available [11]. The requirement for IM administration, which necessitates health care provider administration, can result in decreased parental acceptance due to discomfort and perceptions of a greater potential for harm. Since it is estimated that IPV will remain part of the routine immunization schedule for at least 10 years during the transition away from all OPV [12], a sufficient supply of affordable IPV must be available to meet global needs, which is anticipated to present a serious challenge.
Sabin vaccine (OPV)
Vaccination with live PV leads to its replication in the gut with subsequent induction of both systemic immunity and local antibody production in the gastrointestinal mucosa. Mucosal immunity typically has a secondary beneficial effect in reducing the probability, duration, and concentration of poliovirus shedding in feces upon subsequent challenges with live PV [13]. The effectiveness of trivalent OPV (tOPV) has been documented serologically and clinically in controlling outbreaks. One dose of tOPV produces immunity in more than 95% of recipients in many situations. All countries need rapid access to a supply of OPV for emergent responses when a rapid block of transmission is required. Vaccination with tOPV results in better overall titers than vaccination with IPV. Other advantages of OPV include its ease of administration by non-health care personnel, its low cost, and its acceptance by parents due to the absence of perceptible pain. Interference can occur between the live strains of poliovirus contained in the OPV. Therefore, the mOPV would be expected to have the most robust response, the bOPV exhibits some diminution of response to the separate strains and the tOPV has the most attenuated response to the individual strains. Currently available bOPV has been documented to produce a better immune response against WPV1 and 3, than tOPV [14] with potential to hasten the eradication of the remaining types 1 and 3.
Sabin-like viruses are normally cleared from the stool by one month from the receipt of the vaccine, but concerns exist as to the potential for chronic poliovirus excretion in children with primary immunodeficiency disease (PID). The risk appears to be low for chronic excretion of iVDPV but several studies have documented continued positive fecal tests for immunization strains of PV for periods of months. Of 531 PID patients in Asia and Africa, only 3% tested positive for VDPV excretion for a prolonged period. Although no chronic shedding state has been identified, difficulty still exists in determining whether shedding for as long as 6 months occurred secondary to reduced mucosal clearance in these individuals or from re-acquisition of strains from the environment or through contact with recent OPV recipients.
Although the immunogenicity of OPV in well-nourished, relatively healthy children is usually robust, some concerns have been raised about humoral response in children in underdeveloped countries. In some areas, children have required 10 or more doses of OPV because other circulating viruses and bacteria that infect their gut interfere with the production of an effective immune response [10]. OPV response in infants with malnutrition, diarrhea, and shorter durations of breastfeeding has been shown to be less robust than in infants not experiencing these conditions. Studies have estimated the immunogenicity of a single dose of tOPV in developing countries to be less than half that of developed countries, while IPV immunogenicity appears to remain intact [15]. Zimbabwean infants perinatally infected with HIV also exhibited impaired mucosal and humoral immune responses to OPV and prolonged shedding after their third dose of OPV when compared to children without HIV [16]. However, in contrast, Schoub reported that cell-mediated immunodeficiency such as that produced with HIV did not seem to result in persistent excretion in his population. Mortality in patients with immunodeficiency-related vaccine-derived polio virus (iVDPV) has been reported to be up to 62% [17].
By 2008 WHO had conceded that ultimately, the global eradication of polio was incompatible with the continued use of live-attenuated OPV [18]. Opportunities for mutational change may occur when the attenuated Sabin viral strains circulate between the GI tracts of susceptible individuals in populations where WPV has been eliminated but vaccination rates have fallen resulting in populations highly susceptible to infection. These strains can recombine with other type C enteroviruses within the GI tract and ultimately acquire phenotypical characteristics of neuro virulence and transmissibility comparable to those of WPV. Vaccine viruses that have mutated but still have <1% nucleotide sequence changes from the original vaccine strain in the VP1 gene are termed vaccine-derived poliovirus (VDPV). Rarely these neurovirulent and transmissible mutational changes can result in VAPP. The longer the period between vaccine administration and viral clearing, the more likely mutation is to occur. The polio outbreak in 2015 in Ukraine originated as a VDPV that spread in an environment of low immunization coverage [19].
VAPP is extremely rare and occurs in 1 in 2.7million children receiving their first OPV dose and 1 in 6.9 million subsequent doses. This is approximately 1 case per 2.5 million doses of OPV [20]. Data collected in Latin America between 1992 and 2011 estimated the risk of VAPP to be 1 case in 2.56-4.10 million newborns [21]. The type 3 strain is the most common isolate in VAPP in vaccine recipients but type 2 is the most common isolated in contacts who develop VAPP [6]. By 2011, the type-2 component of OPV was responsible for 90% of the cVDPV cases and 38% of VAPP cases [22]. Transverse myelitis has been estimated to occur once for every 300 million to % billion doses distributed. Because of these rare, but significant, complications, upper and middle-income countries have changed their routine vaccination product to IPV, which induces humoral immunity but does not carry the risk of cVDPV or VAPP. In these countries, the risks of tOPV administration were greater than the risk of contracting polio in their well-immunized populations.
Another concern associated with the use of OPV is the cold-chain required for its use in the field and the difficulties associated with maintaining that chain to ensure stability in tropical and underdeveloped areas. Some studies suggest that vaccine vial monitors that are meant to monitor the cold-chain and therefore protect the potency of OPV by means of a color change are not sufficiently accurate to predict the continued potency of the products [23].
Optimal use of OPV and IPV together
In the decades after the availability of both the OPV and IPV, it became clear to public health officials that he vaccines could be used together to boost population immunity utilizing their respective strengths. Polio could be eradicated rapidly using the greater herd immunity and mucosal titers created by the OPV and once eradicated, the IPV could be administered to maintain the required 90%-95% immunity of a population and minimize the development of VDPV and VAPP during routine immunization in endemic countries. Prior to the switch to bOPV in April 2016, IPV was used to manage the risk of population susceptibility to cVDPV and during the immediate post-cessation period to prevent gaps in protection against polio type 2. IPV can boost immunity to types1 and 3 poliovirus and mitigate the risks of paralysis by priming the population against type 2 and ensuring better immune responses to OPV. Simultaneous administration of IPV and OPV substantially boosts intestinal and systemic immunity beyond that of the OPV alone [24].
IPV boosts mucosal immunity to PV in recipients previously given OPV and helps close the population humoral immunity gap in those who had not seroconverted after OPV. Overall a combined bOPV and IPV schedule is expected to provide higher levels of protection against poliomyelitis than tOPV alone [12]. Data from Oman, Ivory Coast, India, and The Gambia indicates that a single dose of IPV is sufficient to induce seroconversion in 90% of children who remained seronegative after OPV doses. A single dose of IPV can be rapidly boosted by a second dose in cases of threatened or actual outbreak scenarios. Approximately 98% of Cuban infants 4 months of age who did not convert after a single priming IPV converted after receiving a second dose of IPV.
GPEI endgame strategy (2013-2018)
In May of 2015, all WHO Member States endorsed the WHA resolution to complete implementation of the "Polio Eradication and Endgame Strategic Plan 2013-2018". All WHO Member States committed to implementing containment of WPV type 2 in essential lab and vaccine production facilities by 2015 and of type 2 OPV within 3months of global withdrawal in April 2016 [25]. The goals of the plan are the following:
- Certify eradication and containment of all WPV transmission in all WHO regions by 2018.
- Strengthen routine immunization systems by strong OPV vaccination and gradually increased reliance on IPV after interruption of PV transmission has occurred.
- Maintain high levels of population immunity.
- Strengthen networks for accurate and complete detection of circulating polioviruses and strong AFP surveillance.
- Sequential cessation of the use of type-specific OPV to eliminate the risks for VAPP, chronic VDDP infections of immune deficient persons, and outbreaks of cVDPV.
- Develop a plan for mainstreaming essential polio network functions into ongoing public health programs.
Surveillance is a major component of the eradication campaign. Quick case-based AFP surveillance requires a capability for reverse cold-chain from the field to laboratory that is challenging in tropical and impoverished areas. Continued environmental surveillance for poliovirus in sewage or other samples from the local environments is a necessity in current and post- eradication surveillance since AFP surveillance alone is insufficient due to the low percentage of polio-infected patients that go on to develop symptoms. Continued environmental surveillance allows detection of circulating WPV or cVDPV in areas where AFP is not detected as in Israel's silent epidemic in 2013. Israel’s immunization rate using IPV was at least 95% and no cases of paralytic disease were reported, but virologic studies from 25 sewage sites were positive for poliovirus with virologic types indicating introduction from Pakistan and divergence to Egypt, Israel, and Syria [26]. In April of 2016, in agreement with the Endgame goals, tOPV was taken out of use and replaced by the more immunogenic bOPV.
The Sabin strain type 2 was removed from the tOPV since WPV type 2 has not been detected globally since 1999 and is considered eradicated. Significantly, the only type 2 polio cases that have been reported since 1999 have been VDPV. Trigger points have already been set for the continued phasing out of OPV live-attenuated strains as the corresponding WPV is eradicated. The GPEI plan is to complete withdrawal of OPV 3 years after the last WPV is detected globally with a goal to end OPV use entirely by 2020 [27]. The Strategic Advisory Group of Experts on Immunization (SAGE) had also recommended that at least 1 dose of IPV be incorporated into routine infant immunization programs at or after 14 weeks of age by fall of 2015.
Strategies for increasing polio vaccine coverage to obtain the required 80%-90% coverage at district and national levels include national immunization days (NIDS) in scheduled campaigns and subnational days as well (SNIDS) with mop-up activities in response to identified cases of WPV or cVDPV. This strategy allows regions to boost population immunity quickly to multiple targeted age groups including older children or adults. Afghanistan, Nigeria, and Pakistan, the last remaining countries with endemic WPV are using the recommended combination of bOPV during their national and subnational immunization days and IPV within routine immunization schedules.
Challenges and Opportunities
During the last 6 decades, it has become apparent that sustained collaborative support is critical to achieve the ultimate goal of certification of a polio-free world. A lesson learned from smallpox eradication efforts has been that assets from a global health initiative can disappear very quickly once the initial goal has been attained. The need for the Expanded Program on Immunization (EPI) emerged as one of the legacies of the smallpox eradication initiative that has proven its value in providing ongoing assistance to maintain global immunity through immunization over the last 4 decades [28]. Challenges to the complete eradication in the remaining endemic countries of Pakistan and Afghanistan have included continued threats to security in these countriesand the targeted killing of polio vaccinators . These incidents have occurred as recently as January 2016 in Quetta . In Pakistan, bans on vaccination in Taliban-controlled areas have also hindered progress since 2012, Other challenges include cultural issues that limit accessibility to women and their children, refugee migrations, decreased government commitment at various levels, and surveillance gaps. Because of these challenges, full immunization rates for Pakistan in 2013 were at 54% compared with 95% in neighboring Bangladesh [29].
The result has been that in 2013 WHO declared polio an international public-health risk and called for mandatory polio vaccination for everyone travelling to Afghanistan, Nigeria, Pakistan, and several other countries in the region due to the threat of spread from endemic areas. Herd immunity requires high routine coverage of at least 90% for all three doses. Even a relatively brief, 2 year, interruption of tOPV in Romania in the early 1980s was sufficient to result in a contingent of children highly susceptible to infections and resulted in re-implantation of WPV and the emergence of a polio epidemic [6]. In Northern Nigeria politically motivated, rumors resulted in a 10-month ban on tOPV administration. During that period WPV spread to 12 countries from Nigeria and by 2006, 20 previously, WPV- free countries had been reinfected as far away as Indonesia and Yemen. The result was 1475 reported cases of paralytic polio. Effects continued through 2012 when Nigeria was the only country with endemic circulation of WPV in the WHO African Region [30]. In Somalia in 2013 an immunization ban by armed militants led to half a million children going unimmunized for 3 years and the reintroduction of WPV led to a major outbreak 6. In 2015, inadequate vaccine coverage again fueled a polio outbreak in the Ukraine. In 2014, only 50% of Ukrainian children had full immunization coverage against polio. Vaccine coverage through routine immunizations should approach 95% to prevent outbreaks, however, the immunization coverage in Ukrainian children younger than 1-year of age was only 14% in 2015. These rates reflected a public distrust of immunization and an insufficient vaccine supply [19]. The outbreak originated from environmental shedding of VDPV and was fueled by the low vaccination rates.
Timing of IPV doses, now in routine use became more important with the switch to bOPV and was adversely impacted aggravated by the narrow windows of opportunity in some regions. To maximize immunization with potentially infrequent immunizer contacts, the timing of the IPV was determined to be at the same time as the DTP3 immunization, which typically occurs between 3.5 and 6 months of age. SAGE has recommended that the IPV be administered at or after 14 weeks of age. The optimal age decision was based on maximizing IPV immunogenicity when passive immunity was no longer present, the need to protect typ-2 naïve infants against VDPV and VAPP at an early age, and the benefit of vaccination at an age when there is maximal coverage and minimal dropout. The current recommendation was a result of the relative gain in immunogenicity achieve by the administration of IPV together with DTP 3, which outweighed the risk of VDPV type 2 in the first 3 months of life and the potential lowering of vaccination coverage due to dropout between DTP1 and DTP3 immunization encounters [12]. Avoidance of injection-induced polio is also a consideration in countries where the disease is still endemic and OPV is in continued use.
Security threats have highlighted the importance of routine immunization strategies rather than relying on campaigns that may not reach insecure or inaccessible areas [27]. Vaccination campaigns need to be opportunistically planned to take advantage of narrow windows of opportunity such as ceasefires or mass migrations. Clinical trials in Pakistan and Bangladesh showed that 2-3 doses of mOPV with a 1-2week interval were non-inferior at inducing serum-neutralizing antibodies to the same vaccines given with the standard 4-week intervals and that giving IPV simultaneously with OPV substantially boosted intestinal and systemic immunity [24]. Although studies have been done in military recruits [31], there is little information on the immunogenicity of giving more than 3-4 concurrent vaccinations to children living in underdeveloped nations. Faridi reported simultaneous administration of live BCG vaccine and tOPV to term infants prolonged scar formation to BCG, which is regarded as evidence of successful immunization [32].
Refusal of vaccination has been linked with multiple variables including a lack of access to functional radio and TV [33], lower literacy rates, frequency of OPV campaigns and vaccination fatigue [34], the parenteral route of administration [35], lower maternal utilization of antenatal care services, birth order, younger maternal age, and maternal education at less than a primary level [36]. Despite Fatwas supporting vaccination by Grand Imam of Al Azhar and other leading Islamic clerics, support from the Islamic Advisory Group for Polio Eradication of Al-Azhar University, and the International Islamic Authority's support of immunization as a parental responsibility under Islamic law, one third of refusals have been based on religious grounds in Nigeria, India, and Pakistan.
Misinformation has fueled rumors of contamination of vaccines with estrogens, HIV, or pork. Rumors deserve special mention since they have resulted in vaccination boycotts and bans within the decade in Somalia, Nigeria, Pakistan, and Ukraine. The significance of rumors has been underestimated, especially when fueled by local conditions such as the 2011 CIA (US) attempt to obtain DNA samples from the children in Abbottabad, KPP as part of the search for Osama bin Laden. The scheme involved vaccinating children against hepatitis B, and further eroded public trust in immunization. Historical experience with the pharmaceutical industry in the Kano region of Nigeria undermined trust after a bacterial meningitis outbreak 7 years earlier when a pharmaceutical manufacturer allegedly conducted a clinical trial without license or informed consent and several children died [37].
Eradication of polio not only requires the development of high levels of population immunity, but also a network for laboratory and epidemiological surveillance. Almost all countries are susceptible to importation of polio by mobile populations. Because of the low ratio of symptomatic patients to infected patients, the absence of clinical disease is not a reliable indicator of polio transmission. Paralytic polio as a syndrome is indistinguishable from other paralytic syndromes such as Guillain-Barre and therefore case detection and ascertainment requires high surveillance with rapid laboratory follow-up and remains a challenge in underdeveloped countries.
Surveillance networks must have performance capabilities to follow-up on all acute flaccid paralysis (AFP) with two stool samples, 24 hours apart within 14 days of paralysis. Public health networks must have a rapid response plan to institute appropriate interventions to interrupt PV transmission in outbreaks and surveillance for absence of WPV in countries where WPV is considered eradicated. Documentation of this is required for a country or region to be certified as polio-free [38].Polio laboratory networks require a global specialized laboratory, regional reference laboratories and national and provincial laboratories all capable of providing surveillance of AFP cases and viral analysis of ambient environmental cultures. Capabilities must include poliovirus isolation, identification, and intratypic differentiation and sequencing to determine whether WPV or VDPV strains are involved and a general area of origin [39].Since protocols require substantial resources to build, accurate global case surveillance was not achieved until the middle to late 1990s when WHO provided substantial technical assistance to polio endemic countries. As the current situation in Nigeria indicates, there remain areas where surveillance is limited and these "black spots" allow polioviruses to circulate undetected.
Post eradication risks and mitigation
The WHO has planned for stockpiles of over 2 billion doses of all three types of OPV which could be available globally should polio reappear in the post-eradication era. Outbreak response capacity must be maintained, including the management of stockpiles of appropriate type 2-containing vaccines initially and subsequently types 1 and 3 as they are eliminated from the OPV. Countries could potentially need emergent access to all three WPV types as a combination of monovalent, bivalent, and trivalent OPV in addition to the routine use of IPV. Stockpiling of doses and assuring that sufficient manufacturing capabilities are maintained carries with it the risk of inadvertent exposure to live polio strains from manufacturing facilities and storage sites. Planned surveillance and the capability for an emergent response are required in this situation. An escape incident occurred in a production plant in the Netherlands which used WPV for IPV manufacture and caused the asymptomatic infection of a factory worker and his child in 2004. The WHO plan also outlines principles of containment once strain-specific eradication occurs.
In 2006, the Polio Antiviral Initiative (PAI) began as a result of the National Research Council report that concluded that it would be desirable to develop at least two antiviral drugs to supplement vaccination for the control of poliomyelitis outbreaks in a post eradication era. The partnership between the CDC, WHO, FDA, and two other United States-based groups coordinates efforts of drug sponsors in developing polio antivirals. There are currently several drugs in the pipeline, including capsid and protease inhibitors. The situations for which a polio antiviral would be anticipated to be helpful would be for immunodeficient individuals chronically shedding poliovirus, for persons exposed to poliovirus secondary to unintentional occupational exposure, and in conjunction with IPV for communities exposed to a cVDPV in the post-eradication era. It would be anticipated that compassionate use IND applications would be available for use in the first two situations.
Conclusion
We are on the cusp of a polio-free world but continued progress requires cooperation of parents, vaccinators, community and religious leaders, state, provincial, and national government, political leaders, international technical agencies, and major aid donors. Eradicating polio globally requires a high level of political commitment and intense coordination to achieve and then to maintain high levels of population immunity to prevent outbreaks from "black pockets" created by insufficient surveillance. The endgame strategy includes the ultimate withdrawal of attenuated oral vaccine, development of antiviral drugs directed at the poliovirus, and improved routine surveillance in local and regional networks.
For more articles in Global Journal of
Pharmacy & Pharmaceutical Sciences please click on https://juniperpublishers.com/gjpps/index.php
For more about Juniper Publishers please click on https://juniperpublshers.com/index.php



Comments
Post a Comment