Formulation and Evaluation of Chronomodulated Drug Delivery of Montelukast Sodium--Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS GLOBAL JOURNAL OF PHARMACY &
PHARMACEUTICAL SCIENCES
An oral press coated tablet containing Montelukast
sodium was formulated with an outer barrier layer of different
compositions of hydrophobic polymer eythyl cellulose and hydrophilic
polymer hydroxyl propyl methyl cellulose. This press coated tablet was
prepared by using direct compression and wet granulation methods in
order to achieve the predetermined lag time.
Abbreviations: EC: Ethyl Cellulose; HPMC: Hydroxy Propyl Methyl Cellulose; FTIR: Fourier Transform Infra Red; SLS: Sodium Lauryl Sulphate; IR: Immediate Release; ChrDDS: Chrono Modulated Drug Delivery Systems; PCT: Press Coated Tablets
Introduction
Controlled drug delivery systems [1] have acquired a
centre stage in the area of pharmaceutical R &D sector. Such systems
offer temporal &/or spatial control over the release of drug and
grant a new lease of life to a drug molecule in terms of controlled drug
delivery systems for obvious advantages of oral route of drug
administration. These dosage forms offer many advantages, such as nearly
constant drug level at the site of action, prevention of peak-valley
fluctuation, reduction in dose of drug, reduced dosage frequency,
avoidance of side effects and improved patient compliance. In such
systems the drug release commences as soon as the dosage form is
administered as in the case of conventional dosage forms. However, there
are certain conditions, which demand release of drug after a lag time.
Such a release pattern is known as pulsatile release [2-5]. The diseases
currently targeted for chronopharmaceutical formulations are those for
which there are enough scientific backgrounds to justify ChrDDS compared
to the conventional drug administration approach. These include asthma,
arthritis, duodenal ulcer, cancer, diabetes, cardiovascular diseases,
hypercholesterolemia, ulcer and neurological diseases [6,7].
If the organization in time of living system
including man is borne in mind, it is easy to conceive that not only
must the right amount of the right substance be at right place but also
this must occur at the right time. In the last decade numerous studies
in animals as well as clinical studies have provided convincing
evidence, that the pharmacokinetics &/or the drug effects -side
effects can be modified by the circadian time &/or the timing of
drug application within 24 hrs of a day [8]. A pulsatile drug delivery
system that can be administered at night (before sleep) but that release
drug in early morning would be a promising chronopharmaceutic system.
Drug targeting to colon [9] would prove useful where intentional delayed
drug absorption is desired from therapeutic point of view in the
treatment of disease that have peak symptoms in the early morning such
as nocturnal asthma, angina, arthritis.
Circadian rhythms are self-sustaining, endogenous
oscillation, exhibiting periodicities of about one day or 24 hours.
Normally, circadian rhythms are synchronized according to the body’s
pacemaker clock, located in the suprachiasmic nucleus of the
hypothalamus [8]. Asthma is a chronic inflammatory disease of the
airways, characterized by hyper responsiveness to a variety of stimuli.
The role of circadian rhythms in the pathogenesis and treatment of
asthma indicates that airway resistance increases progressively at night
in asthmatic patients. Circadian changes are seen in normal lung
function, which reaches a low point in the early morning hours. The
worsening
of asthma at night commonly referred to as nocturnal asthma
(NA) [10]. A drug delivery system administered at bedtime but
releasing drug during morning hours would be ideal in this case.
Nocturnal asthma is a variable exacerbation of the underlying
asthma condition associated with increases in symptoms, need
for medication, airway responsiveness, and/or worsening of lung
function. Generally, a reduction in peak flow or forced expiratory
volume in one second (FEV1) of at least 20% is implicit in this
definition. Lung function (e.g., peak expiratory flow rate or
FEV1) is usually highest at 4 PM and lowest at 4 AM the latter
time is generally when asthma symptoms are most prevalent. .
Consequently, the administration of a drug formulated in such a
delivery system, i.e. taken at bedtime with a programmed start of
drug release in early morning hours, could offer a more effective
therapy than a typical controlled release drug delivery system,
provided that the most appropriate drugs are administrated
[11].
Pharmaceutical coatings [12] are an essential tool to achieve
the desired formulation of pharmaceutical dosage forms. Coating
techniques mostly used in pharmaceutical industry are aqueous
or organic coating, which present some disadvantages: time
consuming, stability for heat labile and hydrolysis of degradable
drug and polluted environment problem. Thereby, non-solvent
coating is introduced as alternative coating technique to overcome these disadvantages.
For the time controlled release system from compressioncoated
tablets, the amount of the outer shell is a key factor for
controlling the lag time. Higher amount of the outer coating
added would prolong the lag time of drug release [13].
The aim of the present investigation was to develop and
evaluate an alternative, simple, orally applicable one pulse drug
delivery system based on a press-coated tablet preparation.
The PCT investigated in the current study consisting of a
rapidly disintegrating core tablet presscoated by a barrier layer
consisting of varying concentrations of Hydroxy propyl methyl
cellulose (HPMC) and Ethylcellulose (EC). HPMC is a disintegrant
and had been used to cause rapid disintegration of tablets. The
other component of the barrier layer, Ethylcellulose (EC) is a
well-known water-insoluble polymer that has long been used
as a rate-controlling membrane in medication dosage forms to
regulate drug release. Although EC has also been added in tablet
formulations to act as a retarding material, few papers have
focused on the use of EC as a directly compressible excipient.
It was postulated that when the barrier layer was exposed to
dissolution media, the HPMC particles swell and erode [14], a
process which was retarded to varying degrees depending upon
the quantity of EC present, demonstrating that manipulation of
both components controls the erosion rate.
Materials and Methods
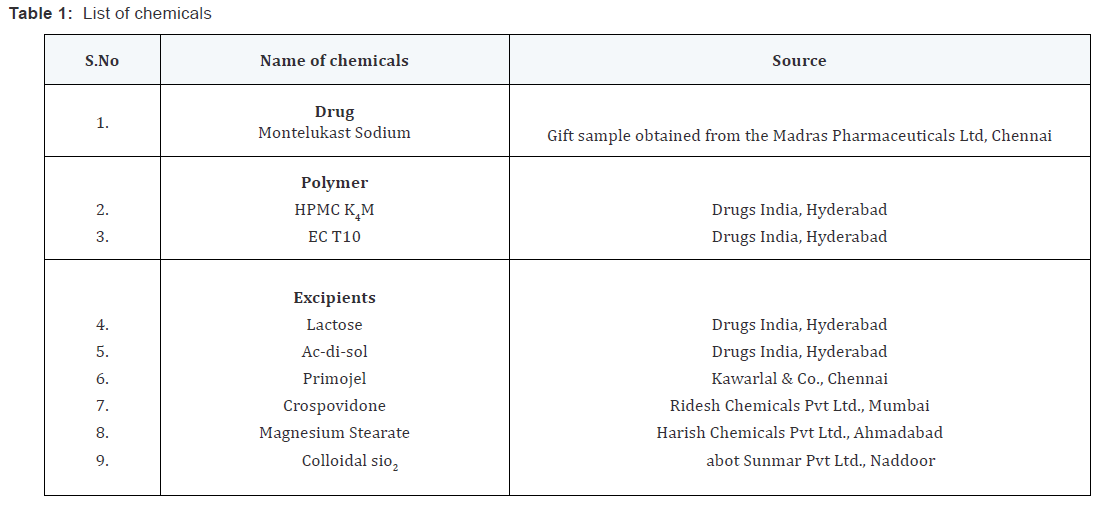
Preparation of core tablets by direct compression
The ingredients depicted in the table except colloidal silicon
dioxide and magnesium stearate were dry blended for 15 minutes
followed by addition of quitted ingredients and dry blended for another 5 minutes. The mixed blend of drug and excipients was
compressed using a single punch CADMACH punching machine
to produce round tablets weighing 100mg with a diameter of
6mm. A minimum of 50 tablets were prepared for each batch.
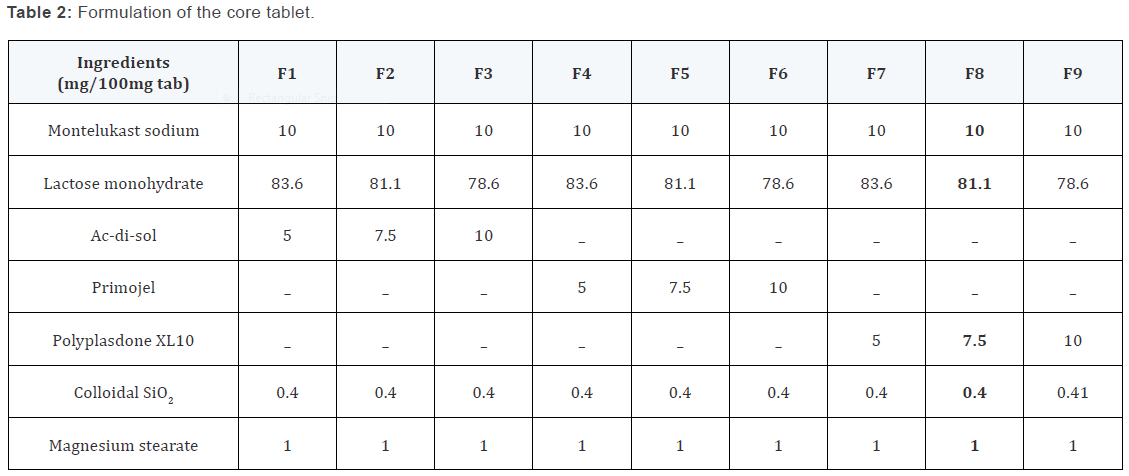
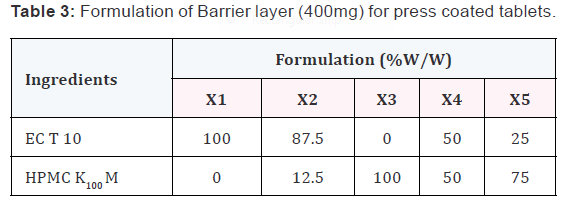
Preparation of press-coated tablets
The core tablets were press-coated with 400mg of prepared
barrier blend as per the mentioned formulas from X1 to X5.
200mg of barrier layer material was weighed and transferred
into a 13mm die then the core tablet was placed manually at the
center. The remaining 200mg of the barrier layer material was
added into the die and compressed.
In vitro drug release study of core tablets
The In vitro release pattern of core tablets was studied as per
method given by Chaudhari SP [15] Release pattern was studied
visually by taking images of the core tablets in a petri plate
containing dissolution medium at the specific time intervals
5sec, 10sec, 15sec. Also the sample was analyzed at 342nm using
a UV spectrophotometer.
In vitro drug release study of press-coated tablets
In-vitro dissolution studies of press coated tablets were
performed at 37 ± 0.5 °C using 0.5% w/v aqueous solution
sodium lauryl sulfate in USP II paddle method at 50 rpm. 5 ml
of filtered aliquot was manually withdrawn at pre-determined
time intervals and replaced with 5 ml of fresh 0.5% sodium
lauryl sulfate solution maintained at the same temperature. The
samples were analysed at 342nm using a UV spectrophotometer.
The lag time and percentage release was determined of the each
formulation.
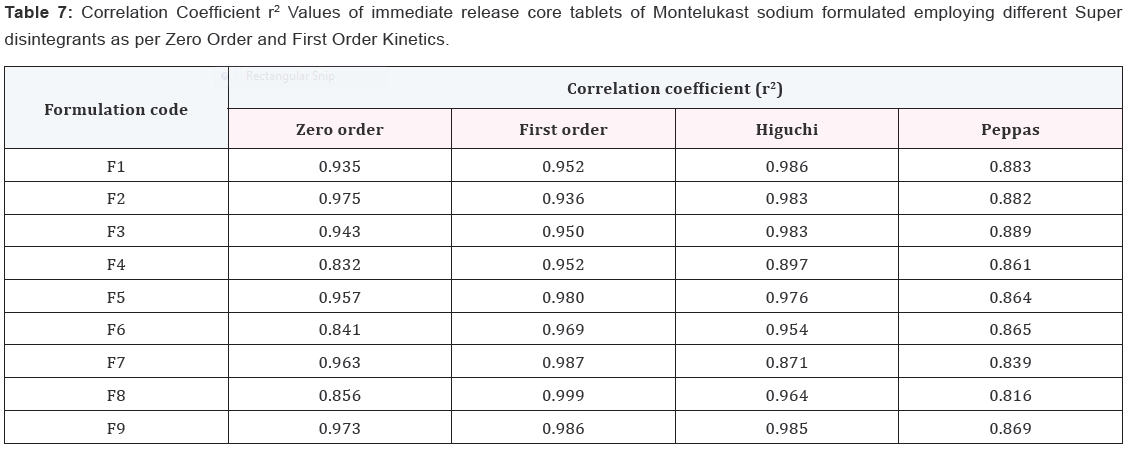
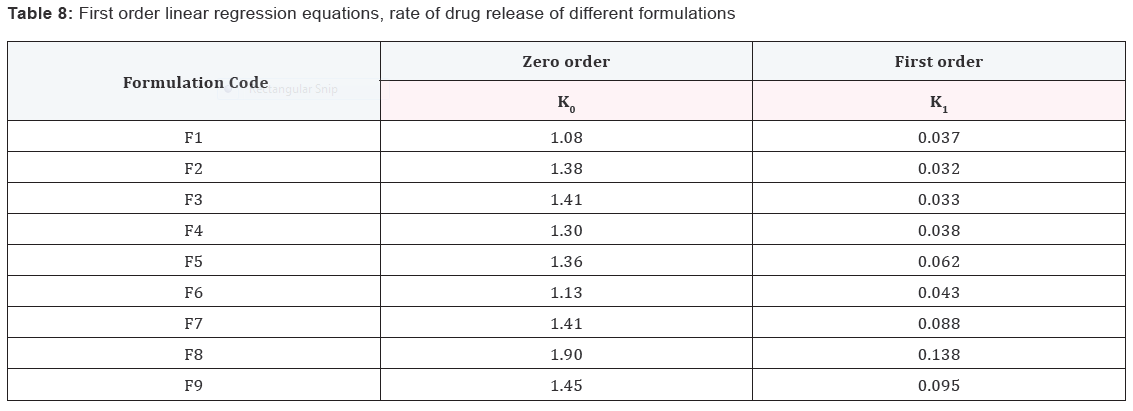
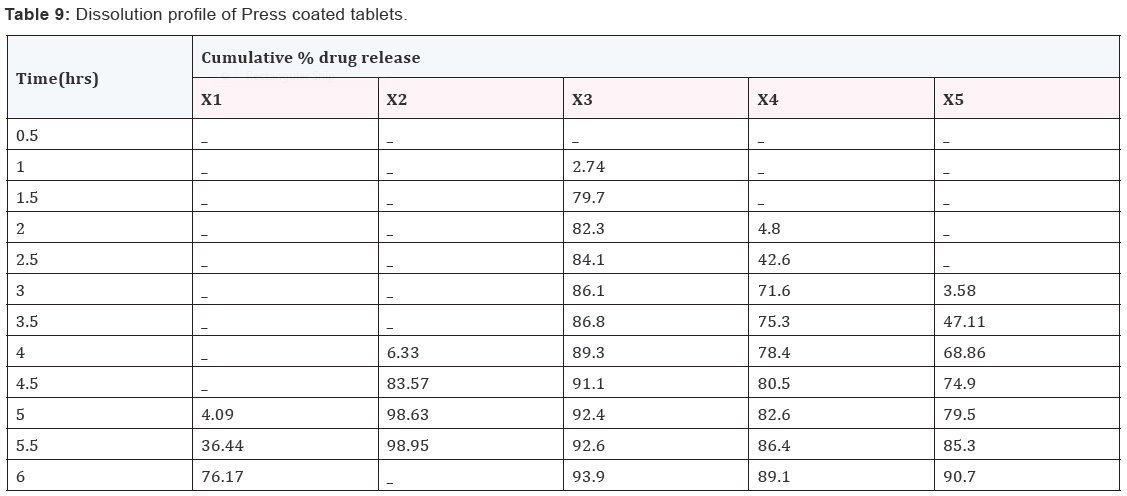
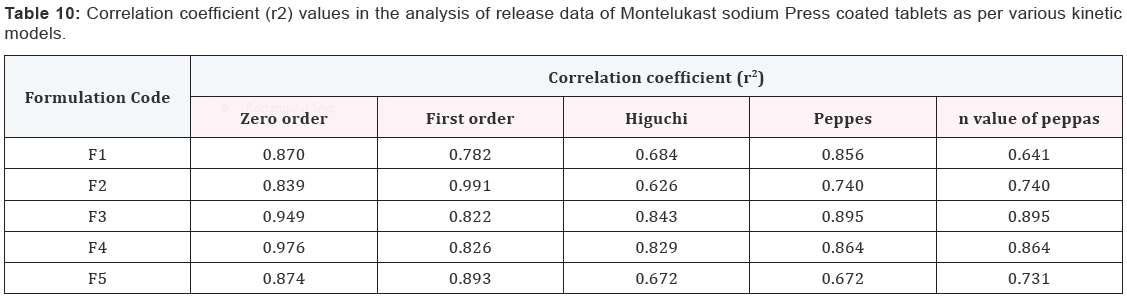
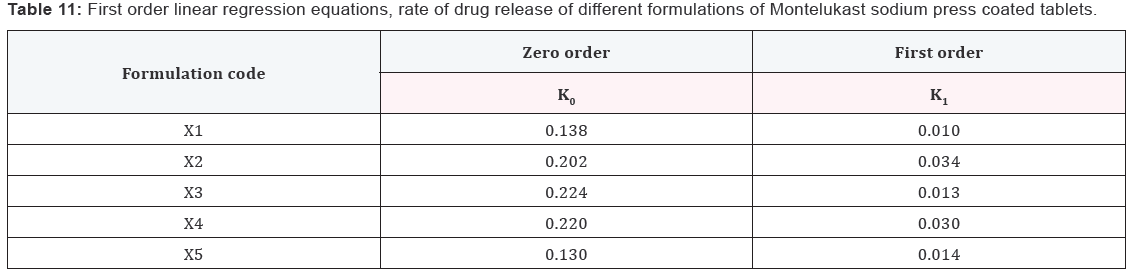
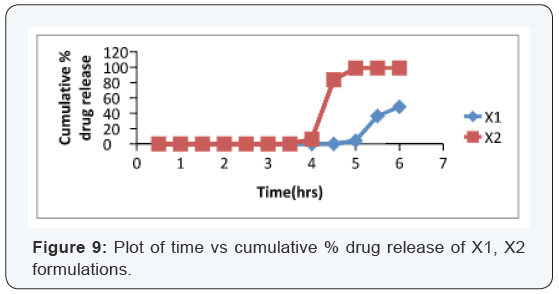
Results and Discussion
Design of Pulsatile release tablet
The pulsatile drug delivery system consisted of inner core
tablet containing drug reservoir and outer coating layer with
combination of water insoluble polymer Ethylcellulose and water
soluble polymer HPMC. Ethyl cellulose was chosen because of its
swelling and rupturable behavior. HPMC was chosen because of
its eroding behavior.
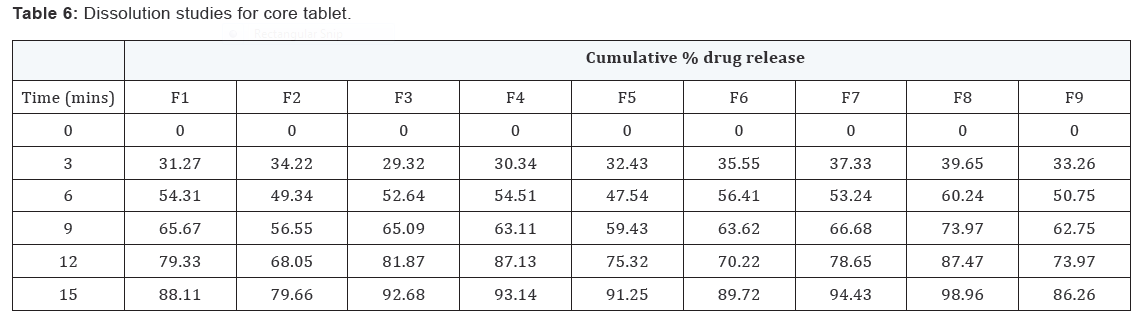
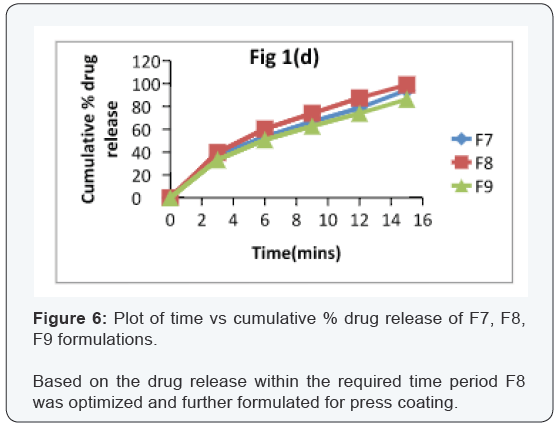
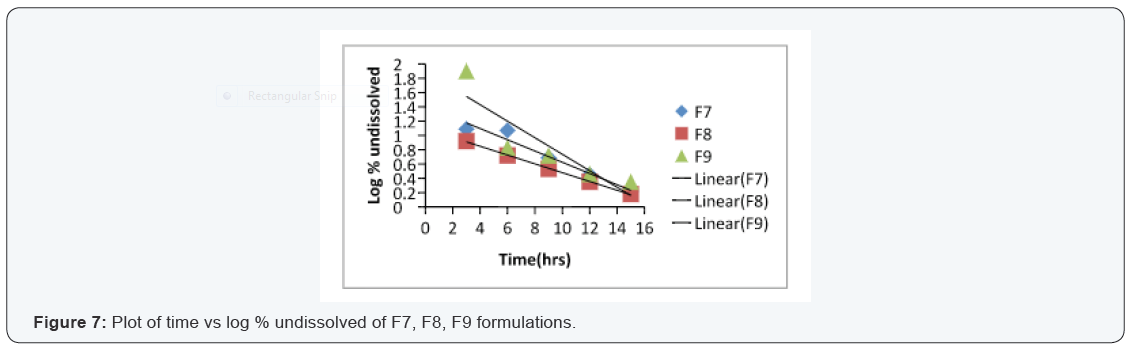
In vitro dissolution of core tablets
The core tablet shows 73.97 % of drug release within 9
minutes upon contact with dissolution medium, core tablet get
erode and release the drug as given in Figure 1.
Analytical methods
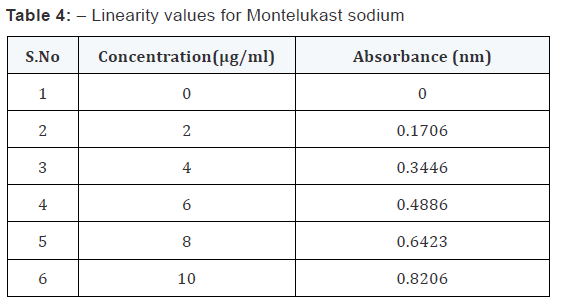
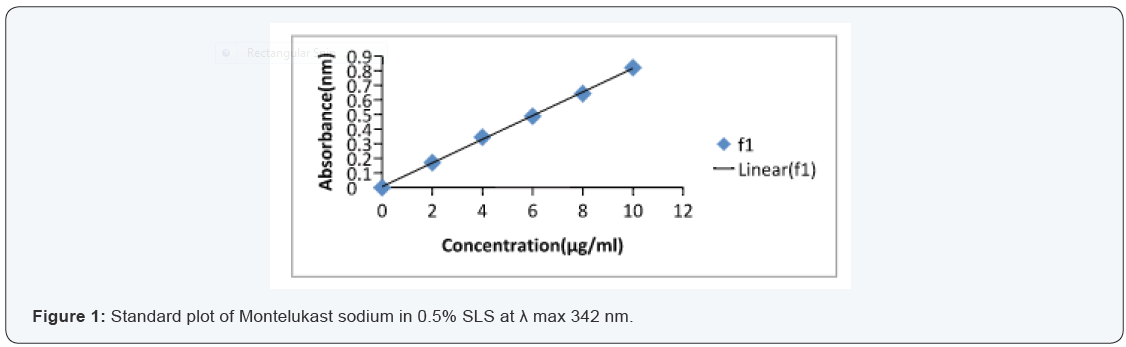
From the standard stock solution (1000 μg/ml), appropriate
aliquot were transferred to series of 10 ml volumetric flasks and
made upto 10 ml with desired solvents so as to get concentration
of 5,10,15,20… or 2,4,6,8… μg/ml. the absorbance of the solution
were measured at 342 nm for Montelukast sodium. This
procedure was performed in triplicate to validate calibration
curve. A calibration curve was plotted.
Compatibility Analysis
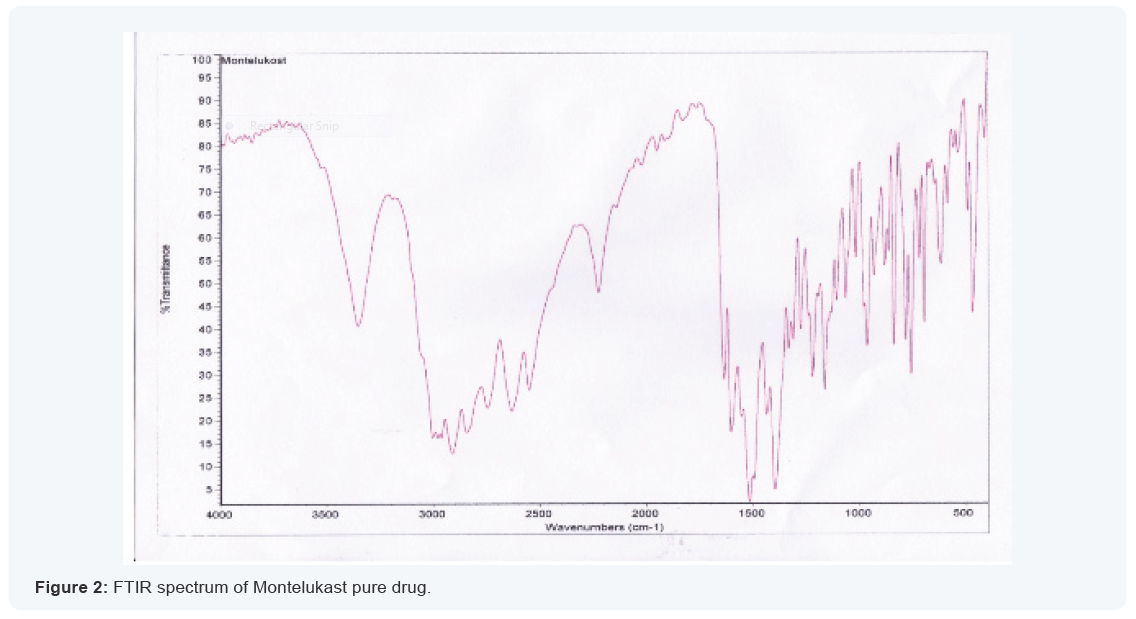
Fourier transform infra-red spectroscopy
FT-IR spectroscopy was employed to ascertain the
compatibility of drugs with polymers. The individual drug and
final formulation were scanned. Both the spectra were compared
for confirmation of common peaks. Montelukast sodium with
polymers showed no significant variation in height, intensity
and position of peaks, suggesting that drug and excipients were
compatible. There is no interaction between drug and polymer.
Hence, it can be concluded that the drug is in Free State and can
release easily from the formulation the spectra are reported in
the Table no.6.3 and Figure 6.2-6.3.



For more articles in Global Journal of Pharmacy
& Pharmaceutical Sciences please click on: https://juniperpublishers.com/gjpps/index.php



Comments
Post a Comment