Development and Validation of UV Spectrophotom- etric Method for Simultaneous Estimation of Omeprazole and Domperidone in Capsule Dosage forms-Juniper publishers
JUNIPER PUBLISHERS-OPEN ACCESS GLOBAL JOURNAL OF PHARMACY & PHARMACEUTICAL SCIENCES
A simple, precise, rapid and accurate UV
Spectrophotometric method has been developed and validated for the
simultaneous estimation of Omeprazole (OMP) and Domperidone (DOM) in
combined pharmaceutical dosage forms. The method was developed by using
methanol as a solvent. Omeprazole exhibits absorption maximum at 301 nm
and Domperidone shows absorption maximum at 287.2 nm in methanol. The
developed method was obeyed Beer Lambert’s law in the concentration
range of 2-12 μg/mL for both OMP and DOM. The accuracy of method was
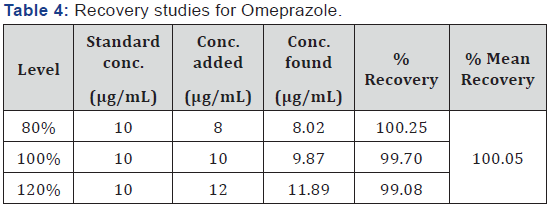
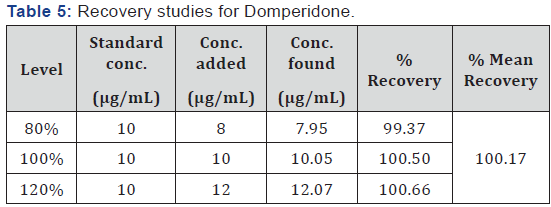
confirmed by recovery studies from capsules at three different levels of
standard additions. %RSD values below 2 for intra-day and inter-day
precision indicates that the proposed method is highly reproducible. The
results of study demonstrated that the proposed method can be applied
to formulation and for routine analysis.
Keywords:Omeprazole; Domperidone; Estimation; Spectrophotometry; Dosage formIntroduction
Omeprazole (Figure 1) chemically
(RS)-5-methoxy-2-((4-methoxy-3,5-dimethylpyridin-2-yl)
methylsulfinyl)-1H-benzo[d]imidazole[1] is a proton pump inhibitor used
is a benzimidazole derivative used in the treatment of dyspepsia, peptic
ulcer disease, gastro esophageal reflux disease, laryngopharyngeal
reflux and Zollinger–ellison syndrome [2]. In peptic ulcers, it
suppresses gastric acid secretion by specific inhibition of the
H+/K+-ATPase in the gastric parietal cell. Domperidone (Figure 2)
chemically 5-chloro-1-{1-[3-(2-oxo-
2,3-dihydro-1H-1,3-benzodiazol-1-yl)propyl]piperidin-4-yl}-2,3-dihydro-1H-1,3-benzodiazol-2-one
is a dopamine antagonist with antiemetic and gastrokinetic properties
used to treat nausea and vomiting. Domperidone facilitates gastric
emptying and decreases small bowel transit time by increasing esophageal
and gastric peristalsis and by lowering esophageal sphincter pressure
[3]. Few analytical methods of HPTLC [4], HPLC [5-7] and UV
Spectrophotometry [8-10] have been reported for the simultaneous
determination of Omeprazole and Domperidone in combined pharmaceutical
dosage forms.
Experimental
Instrument
Shimadzu UV1800 Double Beam UV-Visible
Spectrophotometer was used for spectral studies.
Chemicals and reagents
Standard drug samples of Omeprazole (API) and
Domperidone (API) were obtained from Yarrow Chem Products,
Mumbai, India. The commercial formulation of Omeprazole and
Domperidone capsules were procured from the local market.
Preparation of standard stock solution
The standard stock solutions of OMP and DOM were prepared
separately by dissolving accurately weighed 100 mg of drug in
methanol and volume was made up to 100 mL with methanol to
get concentration of 1 mg/mL From the stock solution prepare
working standard solution of 100 μg/mL concentration in
methanol for both drugs.
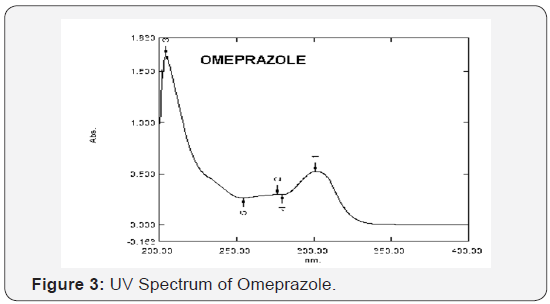
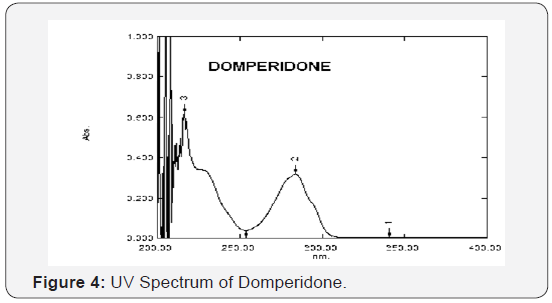
Determination of wavelength of maximum absorbance (λ max)
The standard solutions of both OMP and DOM were further
diluted to get concentration of 10 μg/mL. These solutions were
scanned in the wavelength region of 200-400 nm and the λ max
was observed at 301 nm and 287.2 nm for Omeprazole and
Domperidone respectively. The wavelength spectra of OMP and
DOM in methanol are shown in (Figure 3 & 4) respectively.
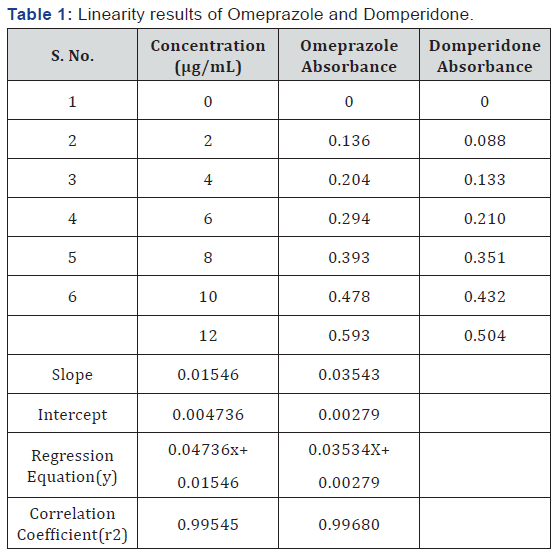
Preparation of calibration curve
Working standard solutions were prepared for the
Omeprazole and Domperidone from the standard solution of 100 μg/mL. Different aliquots were taken from standard stock
solution and diluted with methanol separately to prepare 2
μg/mL, 4 μg/mL, 6 μg/mL, 8 μg/mL 10 μg/mL and 12 μg/mL
solutions respectively. The absorbance values of Omeprazole
and Domperidone were obtained at 301 nm and 287.2
nm respectively. The calibration curves were plotted with
concentrations against absorbance and regression equation was
calculated.
Assay of capsule dosage form
For the estimation of drugs in commercial formulations,
twenty capsules containing 20 mg of Omeprazole and 10 mg of
Domperidone were weighed and average weight was calculated.
An accurately weighed portion of powder sample equivalent
to one capsule weight was transferred into a 100 mL clean dry
volumetric flask containing 70 mL of methanol. The contents of
the flask were sonicated for 10 min and the volume was made up
to the mark with a further quantity of the methanol to get a stock
concentration of Omeprazole and Domperidone. Further pipette
5 mL of the above stock solution into a 10 mL volumetric flask
and the volume was made up to the mark with the methanol.
Results
The present study was carried out to develop a simple,
sensitive, precise and accurate UV spectrophotometric method
for the simultaneous estimation of Omeprazole and Domperidone
in pharmaceutical dosage forms. The wavelength spectrum of
OMP and DOM exhibits at 301 nm and 287.2 nm respectively.
Beer Lambert’s law was obeyed in the concentration range of
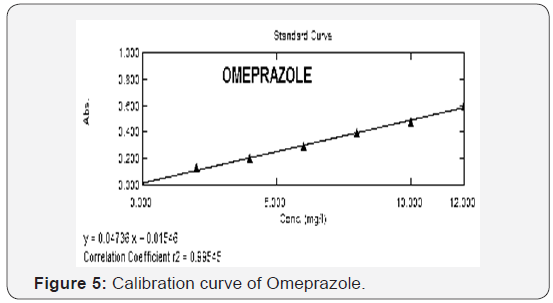
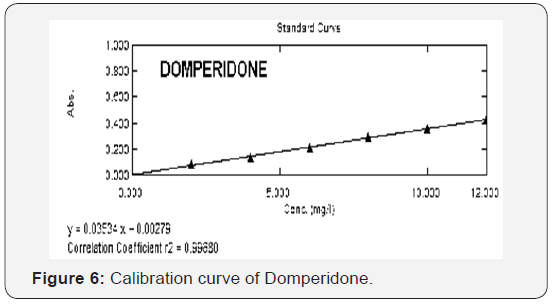
2-12 μg/mL for both OMP (Figure 5) and DOM (Figure 6). The
regression equations for Omeprazole and Domperidone were found to be y=0.04736x+0.01546 and y=0.03534X+0.00279
(Table 1) respectively with a correlation coefficient (r2) of
0.99545 for OMP and 0.99680 for DOM. Precision of the method
was studied by repeated measurements of drug solution and
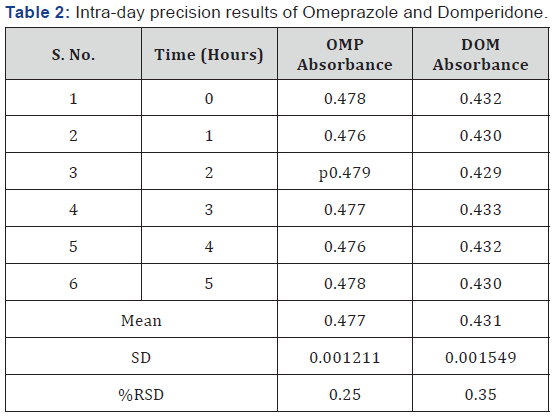
results showed lower %RSD values. The %RSD for intra-day
precision and inter-day precision for OMP were found to be
0.25% and 0.35% respectively. The %RSD for intra-day precision
and inter-day precision (Table 2 & 3) for DOM were found to
be 0.28 and 0.37 respectively. The percent recoveries for OMP
and DOM were found to be 100.05% (Table 4) and 100.17 %
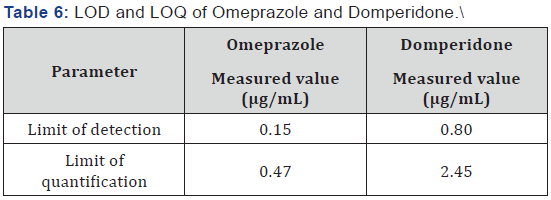
(Table 5) respectively. The limit of detection (LOD) and limit of
quantification (LOQ) for Omeprazole were found to be 0.15 μg/
mL and 0.47 μg/mL respectively. The limit of detection (LOD)
and limit of quantification (LOQ) for Domperidone were found
to be 0.80 μg/mL and 2.45 μg/mL respectively (Table 6). The
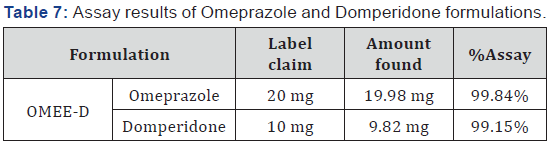
percentage purity for the assay of OMP and DOM were found to
be 99.84% and 99.15% respectively (Table 7).

Conclusion
The UV spectrophotometric method for the simultaneous
determination of Omeprazole and Domperidone in marketed
formulations was developed and validated as per ICH guidelines.
The satisfying recoveries, low correlation coefficient and assay
results confirmed the suitability of proposed method for the
routine quality control analysis for simultaneous determination
of OMP and DOM in pharmaceutical formulations. The %RSD
values for the proposed method reveals high degree of precision
of method .The assay and validation results are satisfactory and therefore the developed method can used for routine analysis of
formulations without interference from excipients.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com
For more articles in Global Journal of Pharmacy & Pharmaceutical Sciences please click on: https://juniperpublishers.com/gjpps/index.php
To know more about Juniper Publishers please click on: https://juniperpublishers.business.site/


Comments
Post a Comment